CD34 is found in bone marrow, blood and umbilical cord. It is used as a marker to identify and isolate stem cells. CD34 expression has been detected in various types of cells, including hematopoietic stem/progenitor cells, multipotent stromal cells (MSCs), muscle stem cells, interstitial cells, fibrocytes, and endothelial stem cells. The main tissue sources of CD34+ cells are bone marrow, mobilized blood and umbilical cord blood. In vitro cultures of hematopoietic stem cells still represent a challenge in research because their fragility prevents them from replicating and also because, in order to differentiate, samples of HSC must be cultured with a highly specific combination of cytokines. Purifying HSC is a challenge, as only 1:10000 bone marrow cells or 1:100000 blood cells is known to be a stem cell.
Bone marrow
Bone marrow is the natural source of CD34+ cells but their isolation is complex and invasive. CD34+ cells are capable of initiating long-term hematopoiesis both in vitro and in vivo. Bone marrow-derived CD34+ cells are a well-characterized population of stem cells and are therefore utilized in the therapeutic re-constitution of bone marrow after radiation or chemotherapy. Recently, CD34+ cells have also been shown to induce therapeutic angiogenesis in animal models of myocardial, peripheral, and cerebral ischemia. The mechanism by which CD34(+) cells promote therapeutic angiogenesis is not completely understood. Expansion of HSCs is a need due to the scarcity of matched donors and difficulties in ex vivo expansion. Expansion of HSCs in vitro through a porous alginate hydrogel-based 3D culture system utilizing bone marrow CD34+ cells has been used.
Bone marrow is obtained from normal donors by bilateral aspirates of the posterior iliac crest. Donors are screened for general health, normal blood counts and infectious diseases prior to collection. Learn more in our Bone Marrow Tech Sheet.
Blood
The population of CD34 in blood is very small and is estimated to be less than 0.5% of all blood cell types. On the other hand peripheral blood is the easiest source of CD34+ cells also because the process is straight forward and non-invasive. Unfortunately, there aren’t large numbers of CD34+ cells in peripheral blood. The obtained number of cells can be increased using cytokine mobilization, which stimulates migration of HSC from bone marrow to the peripheral blood of the donor. Mobilized blood is therefore used as a source for CD34+ cells. Research-grade CD34+ cells isolated from mobilized blood can be used for cell and gene therapy process development. Learn more about CD34+ cells for cell and gene therapy development in an interview with Dr. Manon Destalminil.
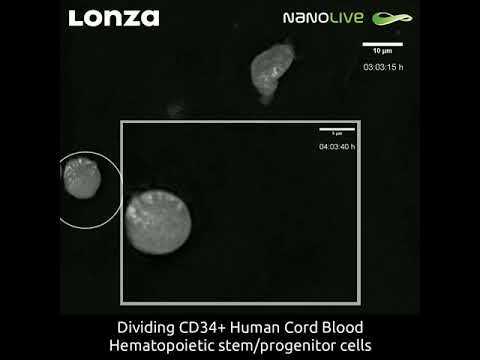
Cord blood
Cord blood is a very accessible source of CD34+ cells. CD34+ HSC isolated from umbilical cord blood present the advantage to efficiently proliferate without losing their ability to differentiate into the several blood cell types. Bone marrow and peripheral blood-derived HSCs allow for large numbers of CD34+ cells to be harvested and transplanted, but they are of limited utility if an HLA-matched donor is unavailable. Cord blood-derived hematopoietic stem cells offer a potential solution, as the cells tend to be more naïve, which may lead to fewer occurrences of host-versus-graft-disease (GVHD) in instances of HLA-mismatch. Cord blood-derived HSCs also pose a major challenge due to the limited number of cells available from any particular donor. Thus, many research labs and clinical trials are currently focused on developing methods for ex-vivo expansion of cord blood-derived HSCs. Learn more about cord blood-derived HSCs and limited cell numbers.