Added to Your Shopping Cart
Certificate of Analysis
Are you looking for...
You might be interested in...
Type in Product name, Keyword or Catalog number to see suggestions.
Looking for a Certificate of Analysis?
Have your catalog and lot numbers ready and click the link.
Find CoA
You searched for "Kinetic Turbidimetric"
Too many choices? Use the filter option on the left to narrow your product listing.
-
PYROGENT®-5000 Kinetic Turbidimetric LAL AssayCatalog #: N384Bulk turbidimetric kinetic LAL Assay for endotoxin testing for 200 tests. Kit contains endotoxin and reconstitution buffer. Assay sensitivity: 0.01 to 100 EU/ml.Sales unit: each
-
LAL Reagent Grade Multi-well PlatesCatalog #: 25-340
96-well individually wrapped microplate specific for endotoxin testing.
Sales unit: units -
LAL Reagent Grade 96-well PlatesCatalog #: 00313405
96-well plates intended for use with LAL-based endotoxin assays
Sales unit: each -
Control Standard EndotoxinCatalog #: 7460L
Endotoxin for PYROGENT®-5000 Bulk Kinetic Turbidimetric LAL, E. coli Strain O55:B5, 25 vials
Sales unit: each -
PYROGENT®-5000 Kinetic Turbidimetric LAL Assay, 100 Test KitCatalog #: N383Bulk turbidimetric kinetic LAL Assay for endotoxin testing for 100 tests. Kit contains endotoxin and reconstitution buffer. Assay sensitivity: 0.01 to 100 EU/ml.Sales unit: each
-
Nebula® Absorbance ReaderCatalog #: 25-365SThe Nebula® Absorbance Reader is a 96-well microplate reader that brings new and improved technology to the laboratory and is optimized to work with WinKQCL® Endotoxin Detection and Analysis Software and our traditional LAL assays such as the PYROGENT® 5000 Kinetic Turbidimetric Assay and the Kinetic-QCL® Kinetic Chromogenic AssaySales unit: each
-
Nebula® Multimode ReaderCatalog #: 25-375S
The Nebula® Multimode Reader is part of the quantitative endotoxin detection system that supports both absorbance and fluorescent based endotoxin detection assays.
Sales unit: each -
Kinetic-QCL® Kinetic Chromogenic LAL Assay, 192 Test KitCatalog #: 50-650U
Kinetic Chromogenic LAL Assay for endotoxin detection for 192 tests, assay sensitivity: 0.005 - 50 EU/mL
Sales unit: each -
Kinetic-QCL® Bulk Kinetic Chromogenic LAL Assay, 2400 Test KitCatalog #: 50-650HBulk Kinetic Chromogenic LAL Assay for endotoxin detection for 2400 tests. Kit contains endotoxin. Assay sensitivity: 0.005 - 50 EU/mLSales unit: each
-
Kinetic-QCL® Bulk Kinetic Chromogenic LAL Assay, 2040 Test KitCatalog #: 50-650NVBulk Kinetic Chromogenic LAL Assay for endotoxin detection for 2040 tests. Kit contains endotoxin. Assay sensitivity: 0.005 - 50 EU/mLSales unit: each
Added to Your Shopping Cart
-
Metabolism_Brochure_CoBrand_FormThis is a co-marketing form for distributors. There is a restricted space on the cover next to the Lonza logo and on the back below the market bar. Art for placement MUST be a PDF. In addition there is space on the back cover for a web address at the top and contact and disclaimers. Please look for the instruction PDF in the literature DB Article number CD-FO006Inst
-
TechSheet - Coronary Artery Endothelial Cell SystemsTechnical information sheet for Clonetics™ Coronary Artery Endothelial Cells
-
TechSheet - Iliac Artery Endothelial Cell System, HIAECTechnical information sheet for Clonetics™ Iliac Artery Endothelial Cells
-
TechSheet - Umbilical Vein Endothelial Cell SystemsTechnical information sheet for CloneticsTM HUVEC cells
-
Instructions for Use - HUVEC Culturing and Angiogenesis Inhibition Assay using Sartorius Incucyte®Protocol for Angiogenesis assay using HUVEC cells
-
Instructions - HUVEC-XL™ Pooled Cell SystemInstructions for culturing Clonetics™ HIVEC-XL™Pooled Cells
-
Diabetes-related Differential Gene Expression in HAECs and ADSCsWith the prevalence of diabetes growing worldwide, the availability of primary human cells from diabetic donors is critical to increase research and knowledge about the disease at a cellular level. In this study, we sought to identify genes differentially regulated in diabetic type 1 and type 2 adipose-derived stem cells (ADSCs) and human aortic endothelial cells (HAECs).
-
Expanded HUVECs for High-throughput ScreeningHUVECs are a good primary endothelial cell type for many in vitro studies and also for several high-throughput screening applications. Consequently, Lonza offers, HUVEC-XL. These cells are expanded to passage-3 from pooled P1-HUVECs and packaged at 10 million cells per ampoule.
-
TechSheet, Instructions - Immo. HMVEC-dTechnical information and instructions for culturing Clonetics® Immortalized Human Dermal Blood Lymphatic Microvascular Endothelial Cells
-
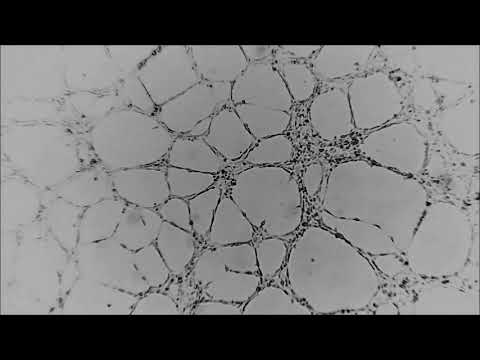 Tube Formation Assay with HUVECHuman Umbilical Vein Endothelial Cells seeded on BME and the formation of endothelial tubes were monitored live with the CytoSMART™ Lux 10X System, the previous version of the CytoSMART™ 2 System. Time-lapse video was recorded for 22 hours at a frame interval of 1 frame every 5 minutes.
Tube Formation Assay with HUVECHuman Umbilical Vein Endothelial Cells seeded on BME and the formation of endothelial tubes were monitored live with the CytoSMART™ Lux 10X System, the previous version of the CytoSMART™ 2 System. Time-lapse video was recorded for 22 hours at a frame interval of 1 frame every 5 minutes.
Product Availability by Store Location
Hours